India's Third Giant Leap
This Could be One of the Biggest Opportunities for Investors
- Home
- Todays Market
- Indian Stock Market News April 30, 2019
Indian Indices Recover; IT and Metal Stocks Witness Buying Tue, 30 Apr Closing
After opening the day on a flat note, Indian share markets witnessed selling pressure throughout the day and recovered during closing hours to end their trading session marginally lower. Sectoral indices ended on a mixed note with realty stocks, telecom stocks and automobile stocks witnessing maximum selling pressure while metal stocks and IT stocks witnessed buying interest.
At the closing bell, the BSE Sensex stood lower by 36 points (down 0.1%) and the NSE Nifty closed down by 7 points (down 0.1%). The BSE Mid Cap index ended the day down by 1.2% and the BSE Small Cap index ended the day down by 1.3%.
Asian stock markets finished on a mixed note. As of the most recent closing prices, the Hang Seng was down 0.6% and the Shanghai Composite was up by 0.5%. The Nikkei 225 was down 0.2%
Speaking of Indian share markets, is the party just about to start in India's growth story?
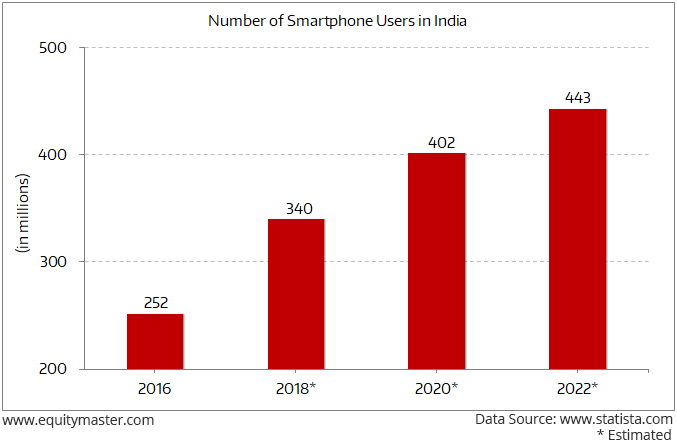
Some indicators certainly seem to suggest so. Take for instance India's smartphone penetration.
Currently, only 20% of India's population has a smartphone.
India's Rapid Increase in Smartphone Penetration
Here's what Tanushree Banerjee wrote about it in today's edition of The 5 Minute WrapUp...
- This is expected to go up to 33% by 2022. With India's population, it is a significant market that will open up soon.
Imagine the number going up to 50% of the population in the near future. And the opportunity that it presents to consumption companies.
Add to this India's population demographic. More than 65% of India's population is below the age of 35.
With higher disposable income, urbanization, the tendency to consume is likely to increase at a rapid pace.
This points to a huge opportunity that lies ahead in India's consumption story.
She believes this is just one of the 50 irreversible trends that will drive the Rebirth of India going forward.
In the news from the banking sector, Kotak Mahindra Bank share price was in focus today as the lender reported its Q4FY19 results today.
The private lender reported a 25.2% year-on-year (YoY) rise in standalone profit at Rs 14.1 billion for the March quarter as provisions fell sharply. Provisions and contingencies declined 44% YoY to Rs 1.7 billion during the quarter under review.
The lender had posted a net profit of Rs 11.2 billion in the corresponding quarter last year. Net interest income (NII) increased to Rs 30.5 billion against Rs 25.8 billion in the year-ago period. Net interest margin (NIM) came in at 4.5%.
Average savings deposits grew by 25% to Rs 762.9 billion against Rs 612.2 billion in the year-ago period. Average current account deposits increased by 14% to Rs 309.3 billion compared with Rs 270.2 billion YoY.
Consolidated profit of the lender increased 13.9% YoY to Rs 20.4 billion against Rs 17.9 billion. The board of the company also recommended a dividend of Rs 0.80 per equity share for the year ended March 31.
In other news, Axis bank is planning to raise Rs 350 billion debt instrument, including in foreign currency to fund business growth. The decision in this regard will be taken at the annual general meeting held on July 20.
Reportedly, the board has already given an approval to raise funds in Indian or foreign currency by issue of debt instruments including but not limited to long-term bonds, non-convertible debentures, perpetual debt instruments and tier-II capital bonds up to an amount of Rs 350 billion.
Last week, the bank reported a net profit of Rs 15.1 billion in the March quarter of FY19, as against a loss of Rs 21.9 billion in the same period last year.
The rise in profit was aided by a fall in total provisions, which more than halved on a year-on-year (y-o-y) basis to Rs 27.1 billion in Q4FY19.
Provisions during the quarter decreased 62.2% y-o-y to Rs 27.1 billion. In the December quarter of FY19, the bank had set aside Rs 30.5 billion as provisions. Post-provisions, the net NPA ratio was at 2.06% in Q4, against 2.36% in the December quarter and 3.4% in the year-ago quarter.
To know more, you can read the detailed results of Axis bank here.
Axis bank share price ended the day up by 0.8%.
Moving on to the news from the pharma sector, Cadila Healthcare received the final approval from the United States Food & Drug Administration (USFDA) to market Bosentan Tablets USP (US RLD-Tracleer Tablets), 62.5 mg and 125 mg and Trientine Hydrochloride Capsules USP (US RLD-Syprine Capsules), 250 mg.
Both the products will be manufactured at the group's formulations manufacturing facility at SEZ, Ahmedabad. Bosentan is used to treat high blood pressure in the lungs (pulmonary arterial hypertension). Trientine is used to treat this inherited condition in people who cannot take penicillamine.
With this approval, the group now has 265 approvals and has so far filed over 350 ANDAs since the commencement of the filing process in FY 2003.
In other news, Cipla and its subsidiary Cipla USA, Inc., have received final approval for the Abbreviated New Drug Application (ANDA) for Ambrisentan Tablets 5mg & 10mg from the United States Food and Drug Administration (USFDA).
Cadila Healthcare share price and Cipla share price ended down by 0.3% and 1.3%, respectively.
For information on how to pick stocks that have the potential to deliver big returns, download our special report now!
Read the latest Market Commentary


Equitymaster requests your view! Post a comment on "Indian Indices Recover; IT and Metal Stocks Witness Buying". Click here!
Comments are moderated by Equitymaster, in accordance with the Terms of Use, and may not appear
on this article until they have been reviewed and deemed appropriate for posting.
In the meantime, you may want to share this article with your friends!