India's Third Giant Leap
This Could be One of the Biggest Opportunities for Investors
- Home
- Views On News
- Jun 1, 2021 - Aurobindo Pharma's Stock Falls After Profits Decline 7%
Aurobindo Pharma's Stock Falls After Profits Decline 7%
Aurobindo Pharma on Monday reported a 7.2% year on year (YoY) fall in consolidated net profit at Rs 8 bn for the quarter ended March 2021.
The company had reported a net profit of Rs 8.6 bn in the same quarter of the previous year.
For the financial year ended March 2021, the company posted a net profit of Rs 53.3 bn. This was up from a net profit of Rs 28.4 bn in the last financial year.
Its consolidated revenue from operations stood at Rs 60 bn, down 2.6% YoY, compared to Rs 61.6 bn reported last year.
The company's revenue from operations was Rs 247.4 bn for the financial year, up 7.3% against Rs 231 bn last year.
The pharma major's earnings before interest, taxes, depreciation, and amortisation (EBITDA) was at Rs 12.7 bn, down 5% over the fourth quarter last year with an EBITDA margin of 21.2%.
The company made good progress on its pipeline effort. It plans to focus more on differentiated and complex generic opportunities. It reached important milestones in the journey during the year.
The company's board approved the transfer of all equity shares held in Aura Cure, a wholly-owned subsidiary of the company, to Eugia Pharma Specialitie, another wholly-owned subsidiary of the company.
Revenue Breakup - Geography and Segment Wise
Formulations: Formulation revenue for the year recorded a growth of 8.4% YoY to Rs 216.9 bn and accounted for 87.5% of total revenues.
For the fourth quarter, formulation revenue declined by 3.6% to Rs 52.1 bn.
US Formulations: In the financial year ended March 2021, US revenue increased by 7.3% YoY to Rs 123.2 bn, accounting for 49.8% of the consolidated revenue.
During the year, Natrol business was divested.
US revenue excluding Natrol increased by 8.2% YoY and revenue in the fourth quarter declined by 4.5% YoY to Rs 28.6 bn, accounting for 47.6% of the consolidated revenue.
Excluding Natrol sales, the company's revenue increased by 5.3% YoY.
It has filed 9 abbreviated new drug applications (ANDAs) including 3 injectables with United State food and drug administration (USFDA) in the quarter. It received final approval for the same.
As on 31 March 2021, on a cumulative basis, the company filed 639 ANDAs with USFDA and received approval for 468 ANDAs including 29 tentative approvals.
The company has launched 19 products during the quarter including 10 injectables.
Europe Formulations: Europe's revenue in the financial year 2021 posted a growth of 2.3% YoY to Rs 60.6 bn, accounting for 24.5% of the consolidated revenue.
For the fourth quarter, the country's revenue decreased by 6% YoY to Rs 15.5 bn due to stock-up at the start of pandemic in the same quarter last year.
The contribution of Europe was 25.9% of the consolidated revenue.
ARV Formulations: Anti-retroviral (ARV) business revenue for the whole year was at Rs 18.6 compared to Rs 12.5 bn, an increase of 48.8% YoY and accounted for 7.5% of revenue.
The increased conversion from tenofovir, lamivudine, and efavirenz (TLE) to tenofovir, lamivudine, and dolutegravir (TLD) across geographies has aided the growth.
ARV business revenue for the quarter was at Rs 4.9 bn compared to Rs 3.8 bn in the same period last year, an increase of 28.7% YoY and accounted for 8.2% of revenue.
Growth Markets Formulations
During the financial year 2021, revenue from growth markets formulations posted a growth of 6.1% YoY to Rs 14.4 bn and accounted for 5.8% of revenue.
Revenue from growth markets formulations in the quarter declined by 18.8% YoY to Rs 3.1 bn and accounted for 5.1% of revenue.
The decline was due to low patient flow to hospitals and pharmacies in certain markets owing to the Covid situation.
API Business: Active pharmaceutical ingredient (API) business clocked a revenue of Rs 30.9 bn and contributed 12.5% to the consolidated revenues during the financial year ended March 2021.
In the March 2021 quarter, API business posted a revenue of Rs 7.9 bn, an increase of 5.1% YoY and contributed 13.2% to the consolidated revenue.
The company filed 1 drug master file (DMF) with USFDA during the quarter.
An active pharmaceutical ingredient means the active ingredient which is contained in medicine. For example, an active ingredient to relieve pain is included in a painkiller. This is called API.
Revenue Breakup for the March 2021 Quarter

Commenting on the company's performance, Mr N. Govindarajan, Managing Director of the company said,
- We are pleased to have ended the fiscal year with steady growth across our key businesses in a dynamic environment affected by the pandemic.
We made good progress on our pipeline efforts to focus more on differentiated and complex generic opportunities and reached important milestones in the journey during the year.
We remain committed to ensure business continuity so that our products reach the patients globally in a timely manner while employee safety and health remains our key priority.
We look forward to execute on our key growth pillars and drive profitable growth.
Aurobindo Pharma, Vaxxinity Seek DCGI Nod to Begin Phase 2/3 of Covid-19 Vaccine UB-612
Aurobindo Pharma said the drug maker and its partner Vaxxinity had approached drugs controller general of India (DCGI) last week seeking permission to conduct phase-2/3 clinical trials of its Covid-19 vaccine UB-612.
N Govindarajan, Managing Director of Aurobindo Pharma, at the company's earnings call on Monday said that the drug maker expects the approval of UB-612 in December or January next year if everything goes according to their plan.
Aurobindo Pharma had entered into an exclusive agreement with US biotech company COVAXX in December 2020 to develop and manufacture UB-612 for India and UNICEF.
COVAXX was renamed as Vaxxinity in April 2021.
UB-612 is based on virus-like particle (VLP) or peptide-based platform.
Aurobindo Pharma has exclusive rights to develop and commercialise UB-612 in India and to UNICEF and non-exclusive rights in other select emerging markets.
The company can supply up to 480 m doses of UB-612 for India and other countries.
Equitymaster's View on the Pharma Sector
We reached out to Tanushree Banerjee, Co-Head of Research at Equitymaster, and editor of the premium stock recommendation service StockSelect, for her view on the pharma sector.
Here's what she has to say...
- The second Covid wave has given a new lease of life to pharma stocks. As the sector continues to invest in capacities for new drugs, the profitability will depend on the companies' ability to seek USFDA approval for the plants.
Or their ability to tie up with MNC pharma for producing variants of their vaccine.
How the Stock Markets Reacted to Aurobindo Pharma's March Quarter Numbers Today
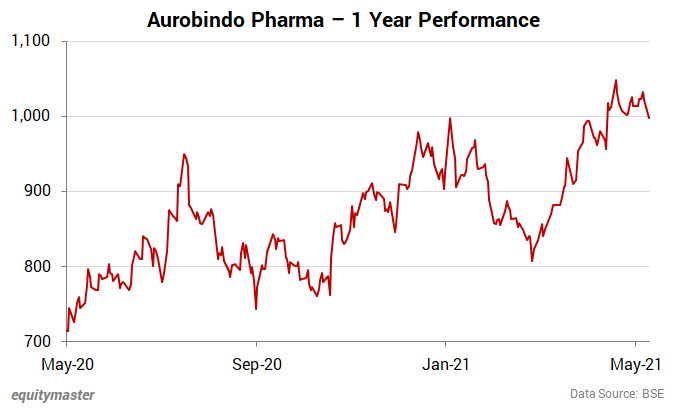
Shares of Aurobindo Pharma opened the day at Rs 1,000 on the BSE and Rs 1,003 on the NSE.
Aurobindo Pharma share price closed at Rs 970.4 (down 2.7%) on the BSE and Rs 970.3 (down 2.8%) on the NSE.
At its current price, it is trading at a P/E of 10.5.
The share touched its 52-week high of Rs 1,063.8 and 52-week low of Rs 703.7 on 11 May 2021 and 28 May 2020, respectively.
Over the last 30 days, the Aurobindo Pharma share price is up 0.2%. Over the last one year, the company's share price is up 35%.
About Aurobindo Pharma
Aurobindo Pharma (APL) one of the world's top 5 manufacturers of semi synthetic penicillin was incorporated on 26 December 1986 as a private limited company.
The company is into developing, manufacturing, and marketing active pharmaceutical ingredients (APIs also referred as bulk actives) intermediates and generic formulations.
Its robust product portfolio is spread over 6 major product areas encompassing (antibiotics, anti-retro, virals CVS, CNS gastroenterological, and anti-allergics) with around 65 APIs in the non-antibiotics and over 55 APIs in the antibiotic segment.
APL is running with 14 manufacturing plants across the world and an extremely well-equipped research and development (R&D) facility.
Aurobindo Pharma has identified international operations also catering to over 100 countries.
For more details about the company, you can have a look at Aurobindo Pharma factsheet and quarterly results on our website.
You can also compare Aurobindo Pharma with its peers.
Aurobindo Pharma vs Divi's Laboratories
Aurobindo Pharma vs Alembic Pharma
Aurobindo Pharma vs Glenmark Pharma
To know what's moving the Indian stock markets today, check out the most recent share market updates here.


Equitymaster requests your view! Post a comment on "Aurobindo Pharma's Stock Falls After Profits Decline 7%". Click here!
Comments are moderated by Equitymaster, in accordance with the Terms of Use, and may not appear
on this article until they have been reviewed and deemed appropriate for posting.
In the meantime, you may want to share this article with your friends!